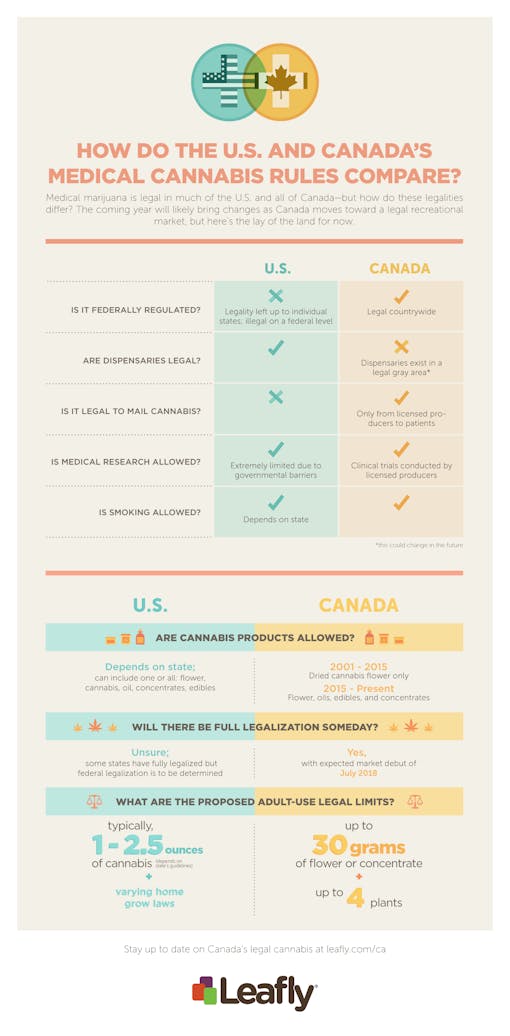
As Canada prepares to legalize cannabis countrywide, many have wondered how its medical marijuana program compares to the United States’. Here are five key similarities and differences between each country’s medicinal cannabis guidelines.
Federal vs. State Regulation
The biggest notable difference between the Canadian and American medical marijuana systems is that Canada’s program is federally regulated, while America relies on separate state programs that vary significantly. Cannabis in the United States is prohibited at a federal level, which means each state must set up its own program individually, establishing possession limits, restrictions on cultivation, and nonstandard rules.
Canada, conversely, federally legalized medical cannabis in 2000, and the program has since undergone multiple incarnations. The Medical Marihuana Access Regulations (MMAR) was first established in 2001 and allowed patients to grow cannabis with an authorization. The system was revamped in 2013 and renamed the Marihuana for Medical Purposes Regulations (MMPR), which got rid of personal production permits and established a system of licensed producers to grow and sell cannabis. On August 24, 2016, the system underwent its final transformation into the Access to Cannabis for Medical Purposes Regulations (ACMPR).
Shipping Marijuana vs. Shopping In-Store
Health Canada is tasked with overseeing the medical marijuana program and regulating the licensed cannabis producers that cultivate cannabis to be shipped to patients. Canada is unique in that not only is cannabis allowed to be shipped to medical marijuana patients, it’s actually required. It is not legal, however, for private patients to ship cannabis to each other; the shipment can only come from a licensed producer. Although medical marijuana dispensaries are fairly prominent in Canada, they exist in a legal gray area and are often subject to raids.
In the United States, it is prohibited to ship or transport any federally restricted substance through the US mail system or across state lines. To do so is to risk a federal felony charge from the U.S. government. Patients may not order cannabis online or have it legally shipped to them; instead, they may visit a state-regulated medical marijuana dispensary to purchase cannabis with a valid state-issued medical marijuana authorization.
Medical Cannabis Research
The opportunities for federal clinical trials on the effects of cannabis as treatment for certain medical conditions are complicated in both Canada and the United States, and earning the right to study cannabis is not a particularly simple process.
Although Canada legalized medical marijuana in 2000, Health Canada largely placed the responsibility of the program on individual doctors. When the system was revamped in 2013, the focus was placed on licensed producers. With the introduction of LPs, it became clear that the companies had a vested interest in studying cannabis in clinical trials, and these producers have spurred much of the new research, including trials for the efficacy of cannabinoids on seizure disorders, PTSD, and in chemotherapy-induced nausea and vomiting.
Medical marijuana research in the United States faces several major barriers from the government, including the National Institute on Drug Abuse (NIDA), the Food and Drug Administration (FDA), and the Drug Enforcement Administration (DEA). For example, Dr. Sue Sisley has spent seven years jumping through hoops for a clinical trial on the efficacy of cannabis for PTSD. She received a $2.1 million grant from the Colorado Department of Public Health and Environment, but needed approval from NIDA to gain access to the only medicinal cannabis available for federal studies. She got NIDA’s approval in March 2014, but was unable to access the cannabis for another three years. When she did gain access, it was clear that the marijuana was not up to par–it contained mold and scant amounts of THC. The trial has been slow, but is making progress and data should be available by January 2019.
Cannabis Concentrates (and Other Non-Smokeable Products)
Curiously, Canada only allowed “dried marijuana plant material” for the first 15 years of the program’s existence. The regulations did not mention or acknowledge any other cannabis products, such as tinctures, oils, edibles, and pills. It took a ruling from the Canadian Supreme Court that the restriction “unjustifiably violates the guarantee of life, liberty and the security of the person” for oils, edibles, and cannabis concentrates to be permitted. Licensed producers are still not permitted to sell edibles, but they do sell cannabis oils and concentrates, which patients are permitted to use to infuse their own homemade edibles.
Shop highly rated dispensaries near you
Showing you dispensaries nearThe United States, on the other hand, has had a different journey towards approving cannabis concentrates. The process has been largely dependent on when the state legalized medical marijuana. Many states legalized medical cannabis before concentrates and oils were popularized, but now, not only are they commonplace, it’s increasingly clear what a positive impact oils and edibles are for medical purposes. For patients who suffer from debilitating symptoms, concentrates offer longer-lasting effects are have fewer detrimental health effects than smoking flower.
Pending Legalization
Colorado and Washington may have beat Canada to the punch with legalization on a small scale, but Canada’s proposed Cannabis Act would legalize cannabis for adult use nationwide. Anyone 18 years of age and older would be allowed to possess up to 30 grams of cannabis flower or concentrate, and grow up to four plants for personal use.
The United States has not set any date or timeline for federal legalization, but the states that have fully legalized established the minimum age at 21 years old, and the legal possession limit ranges from one ounce to 2 ½ ounces of cannabis. Home cultivation still varies from state to state; for example, in Washington state, it’s legal to possess up to an ounce of cannabis, but home cultivation is not permitted by non-medical patients.